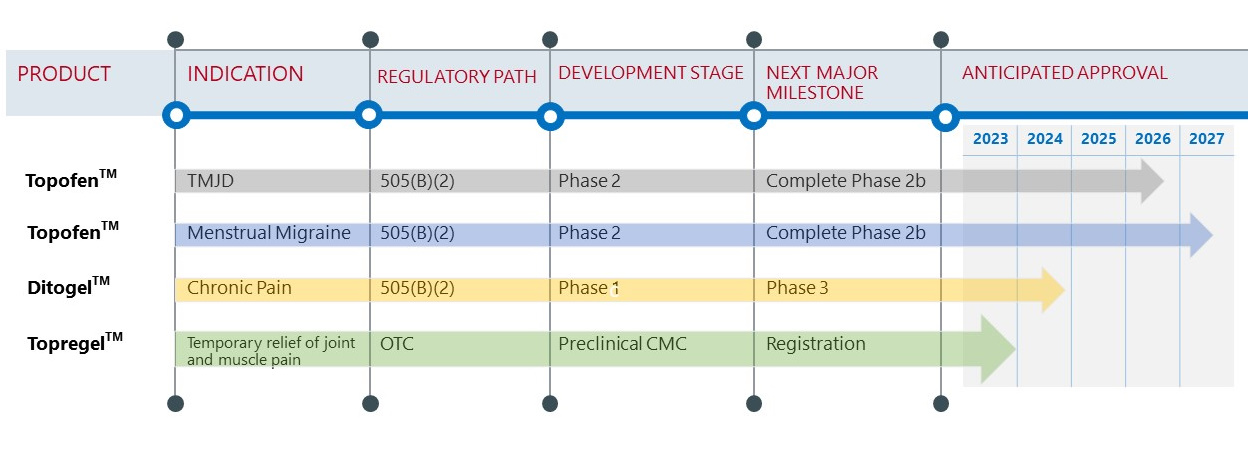
Achelios has a Pain-Focused Pipeline

Achelios is developing several proprietary topical options with a potentially better safety profile when compared to oral dose formulations. Our novel delivery approaches are designed to offer the patient improved efficacy and safety by minimizing systemic drug exposure and off-target effects.
- Our pipeline consists of novel, non-opioid therapies currently in development to treat a broad array of chronic pain indications.
- Our pipeline is uniquely positioned to address the limitations of currently available therapies by providing safe, effective, and long-lasting pain treatment options.
Achelios’ lead development product is Topofen™ for the treatment of temporomandibular joint pain and migraine. Topofen™ is formulated using our topical Achetogel™ topical delivery platform. In preliminary clinical experiments, Topofen has shown efficacy in treating TMJD and migraine pain. Topofen is currently being evaluated as a treatment for chronic temporomandibular pain and as a prophylactic agent in menstrual and episodic migraines.
OTC Product For Pain Relief in 2023